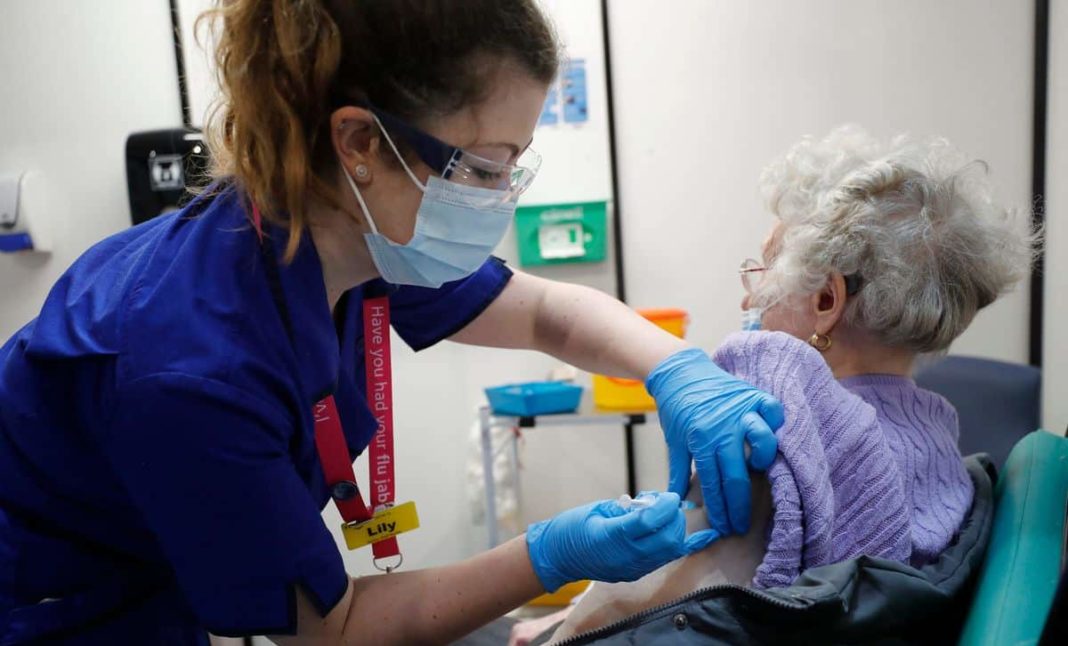
The Pfizer vaccine was approved for use last week by the regulator and was rolled out in hospitals on Tuesday
UK regulators have issued a warning to those people who have had a history of “significant” allergic reactions and have said that they should not currently be receiving the Pfizer/BioNTech COVID-19 vaccine.
This news comes after two NHS staff members who had received the vaccine yesterday experienced allergic reactions following the jab.
They had reportedly developed the symptoms of an “anaphylactoid reaction” shortly after being injected with the Pfizer vaccine, and are now understood to be recovering.
This comes after a 90-year-old grandmother-of-four became the first patient in the world to receive Pfizer’s coronavirus vaccination outside of a trial. Mrs Keenan, who lives in Coventry but is originally from Enniskillen, Northern Ireland, was given the vaccine by nurse May Parsons at Coventry’s University Hospital.
The two NHS staff members are among the thousands of people in the nation who received the first dose of the Pfizer vaccine yesterday, as the biggest vaccination programme in the history of the UK got under way.
The UK became the first country across the globe to approve the Pfizer vaccine last week, with care home workers and hospital patients, as well as NHS staff, among others, being the first to receive the jabs.
Known as the MHRA, The Medicines and Healthcare products Regulatory Agency has given precautionary advice to NHS trusts throughout the country that anyone who has had a history of “significant” allergic reactions to medicines, food or other vaccines should not receive the Pfizer vaccine.

In a statement, Pfizer UK said: “We have been advised by MHRA of two yellow card reports that may be associated with allergic reaction due to administration of the COVID-19 BNT162b2 vaccine.”
“As a precautionary measure, the MHRA has issued temporary guidance to the NHS while it conducts an investigation in order to fully understand each case and its causes. Pfizer and BioNTech are supporting the MHRA in the investigation.”
“In the pivotal phase 3 clinical trial, this vaccine was generally well tolerated with no serious safety concerns reported by the independent Data Monitoring Committee. The trial has enrolled over 44,000 participants to date, over 42,000 of whom have received a second vaccination.”
This comes after there are fears that some of the poorer countries in the world could be left behind as richer nations “hoard” more doses of the COVID-19 vaccines than they actually need.
The head of the MHRA, Dr June Raine, told a joint select committee hearing that “real-time vigilance” would continue even now that the COVID-19 vaccine had been deployed.

“Even last evening we were looking at two case reports of allergic reactions.”
“We know from the extensive clinical trials that this was not a feature, but if we need to strengthen our advice now that we have had this experience in vulnerable populations, the groups selected as a priority, we get that advice out immediately.”